 Membrane Biochemistry The current conceptual framework of metallochemistry is inadequate to explain how membrane transporters acquire zinc against thermodynamic gradients while maintaining rapid zinc mobility across the membrane barrier with extraordinary selectivity over similar metal ions several orders of magnitude more abundant in vivo. To address this fundamental question, our research has focused on a complementary pair of zinc-transporting model proteins, YiiP and ZIPB. YiiP is a bacterial homolog of ZnT83, while ZIPB is homologous to a completely different class of mammalian zinc transporters responsible for cellular zinc uptake and intracellular zinc mobilization4. Both YiiP and ZIPB share a common metal selectivity for zinc, but utilize distinct energetic mechanisms to move zinc in opposite directions across the cytoplasmic membrane. As such, YiiP and ZIPB build around the same zinc coordination chemistry, but exploit unique structural designs to achieve diverse functions. By dissecting and analyzing the structures and functions of YiiP and ZIPB, we hope to elucidate the inner workings of zinc transporters at the interface of protein biochemistry and zinc metallochemistry.
Membrane Biochemistry The current conceptual framework of metallochemistry is inadequate to explain how membrane transporters acquire zinc against thermodynamic gradients while maintaining rapid zinc mobility across the membrane barrier with extraordinary selectivity over similar metal ions several orders of magnitude more abundant in vivo. To address this fundamental question, our research has focused on a complementary pair of zinc-transporting model proteins, YiiP and ZIPB. YiiP is a bacterial homolog of ZnT83, while ZIPB is homologous to a completely different class of mammalian zinc transporters responsible for cellular zinc uptake and intracellular zinc mobilization4. Both YiiP and ZIPB share a common metal selectivity for zinc, but utilize distinct energetic mechanisms to move zinc in opposite directions across the cytoplasmic membrane. As such, YiiP and ZIPB build around the same zinc coordination chemistry, but exploit unique structural designs to achieve diverse functions. By dissecting and analyzing the structures and functions of YiiP and ZIPB, we hope to elucidate the inner workings of zinc transporters at the interface of protein biochemistry and zinc metallochemistry.
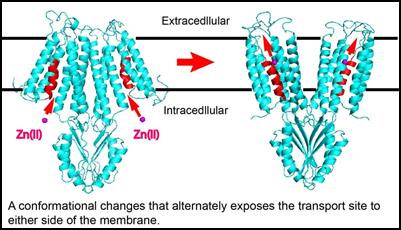
Biophysics and Structural Biology Zinc mobility makes zinc transporters fundamentally distinct from zinc metalloenzymes where zinc coordination is permanent in catalytic centers. In contrast, zinc binding to membrane transporters is transient and coupled to a vectorial movement across the membrane barrier. The coordination chemistry revealed in crystal structures of metalloenzymes is not applicable to membrane transporters. To fill in this critical knowledge gap, we determined the crystal structure of YiiP at atomic resolution5-6. The YiiP structure suggests how zinc coordination chemistry is coupled with protein dynamics to allow a rapid zinc passage across the membrane. The timescale of zinc transport is millisecond, vastly faster than metalloproteins that typically take hours to release bound zinc7-10. We have developed time-resolved biophysical approaches to follow metal transport and monitor protein conformational changes in real time by stopped-flow spectrofluorimetry11 and x-ray mediated hydroxyl radical labeling followed by bottom-up mass spectrometry11. These time-resolved experiments suggested a dynamic mechanism by which a physiological proton gradient, zinc coordination chemistry and water nanofluidics are orchestrated to overcome the activation barrier to zinc efflux through a highly conserved hydrophobic gate. Our current research is focusing on crystallization and structural determination of complementary zinc transporting proteins and exploring zinc-mediated protein conformational changes.
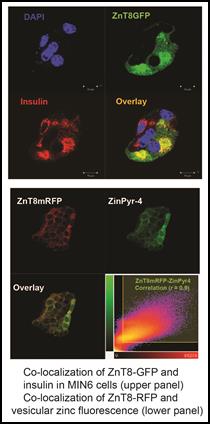
Cell Biology The mechanistic framework of zinc transport is likely conserved from bacteria to humans as demonstrated by a comparative study of YiiP and human ZnT8/ZnT52. Mammalian zinc transporters achieve greater functional diversity and plasticity by extending conserved core functions through: (i) fine tuning the transport activity by post-translational modifications, (ii) regulating protein abundance and subcellular localization, and (iii) networking with intracellular zinc trafficking pathways via protein interactions. As a result, mammalian zinc transporters give rise to distinct subcellular zinc distributions with sharp transmembrane zinc gradients among intracellular organelles. The temporal and spatial dynamics of subcellular zinc distributions provide crucial cellular signaling opportunities, but also challenge intracellular zinc homeostasis with broad disease implications such as diabetes. To understand the functions of pancreatic zinc transporters, we have purified human ZnT8 for direct biochemical characterization and crystallization. We also have established in vivo zinc imaging techniques using zinc fluorescence indicators, genetically-encoded zinc sensors and direct metal imaging by synchrotron-based x-ray fluorescence microscope. Clonal pancreatic cell lines with targeted manipulations of zinc transporters have been established in our lab, thus subcellular zinc distribution may be correlated to the molecular functions of specific zinc transporters.
Proteomics Zinc uptake and efflux transporters, by virtue of their strategic locations at the entry and exit points of various cellular zinc trafficking pathways, form major interaction nodes within the cellular metallointeractome. Specific protein interactions are envisioned to govern zinc trafficking to competitive protein sites against a repertoire of similar transition metal ions. We are developing proteomic tools to identify zinc metalloproteins that directly bind and regulate zinc transporters. Identification of the interacting partners of zinc transporters is the first step toward solving a long-standing cell biology mystery: how zinc ions selectively populate their protein targets in the right subcellular locations, at the right time, and in the right amount.
Biomedical Applications One of the recent breakthroughs in basic diabetes research is the discovery of a human zinc transporter (ZnT8) as a major risk factor for type-2 diabetes (T2D))a. ZnT8 is also a major autoantigen in type-1 diabetes (T1D)b. Loss-of-function mutations in ZnT8have been found to confer protection against T2Dc, suggesting that ZnT8 inhibition could be a potential therapeutic strategy. Recently we have successfully purified recombinant human ZnT8. The availability of the purified ZnT8 protein enables the development of monoclonal antibodies (mAbs) against intact ZnT8 in its native conformations. Unlike conventional mAbs targeting linear peptide epitopes, the conformation-specific mAbs are expected to have superior binding specificity and the ability to inhibit ZnT8 by locking it to a fixed conformational state. The ZnT8 mAbs potentially could be used for early detection of T1D autoantibodies (ZnT8 is a major antigen in T1D autoimmune response), and for the treatment of T2D (Loss-of-function mutations in ZnT8 protect against T2D)c.